Periodic Table Element Properties: Unlocking Material Behavior
Understanding the periodic table is fundamental to materials science. It's a roadmap guiding the design and prediction of material properties. This article explores key element properties, highlighting areas of established understanding and areas requiring further research. We’ll examine how subtle atomic differences significantly impact material behavior and provide practical guidance for materials selection and property prediction. For a complete list of chemical elements, see this helpful resource.
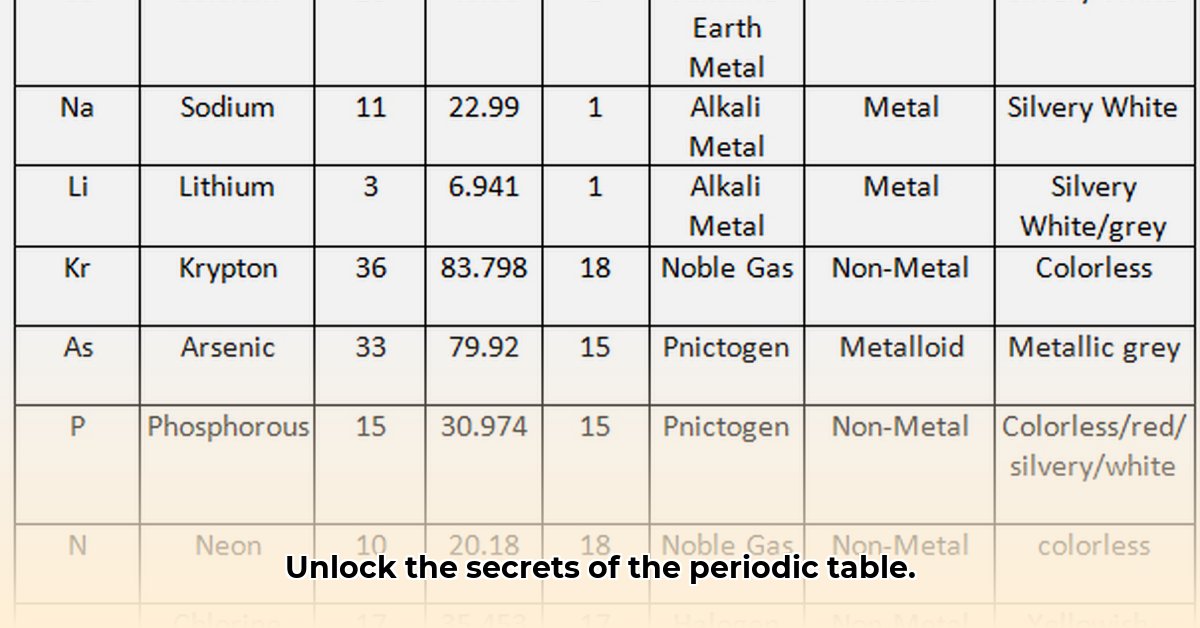
Atomic Weight: Mass at the Subatomic Scale
Atomic weight, the average mass of an element's atom, is a foundational property. It influences a material's density, though not exclusively. Heavier elements generally correspond to denser materials (e.g., gold vs. aluminum). However, crystal structure and atomic bonding significantly modify this relationship. Precise atomic weight determination is crucial for accurate material modeling and property prediction. "The accuracy of our models is critically dependent on the precision of atomic weight data," notes Dr. Anya Sharma, Professor of Materials Science at MIT.
State of Matter at 273K: A Snapshot of Physical Behavior
The state of matter (solid, liquid, gas) at 273K (0° Celsius) provides a key insight into an element’s behavior under standard conditions. Solid elements typically exhibit strong atomic bonds forming rigid structures, while liquids show weaker bonds allowing for greater atomic mobility. Gases, characterized by the weakest bonds, have highly mobile atoms. This property directly impacts material applications; a liquid at room temperature is unsuitable for high-temperature applications, whereas a solid might be ideal for structural components. How can we reliably predict an element's phase at 273K? This remains a significant challenge for some elements.
Data Discrepancies and Knowledge Gaps: Challenges and Opportunities
While considerable agreement exists on atomic weights, uncertainties persist, particularly regarding the state of matter at 273K for certain elements, especially rare or less-studied ones. Some elements' 273K states are marked as "unknown." This presents both a challenge and an opportunity. "Uncertainties in element properties represent significant knowledge gaps. Addressing them through experimental and computational work is critical," states Dr. Kenji Tanaka, Lead Researcher at the National Institute for Materials Science, Japan.
Addressing Uncertainties: Research Strategies for the Future
Filling these knowledge gaps requires a multi-pronged approach. This includes:
- Advanced Experimental Techniques: Developing improved experimental methods, particularly for highly radioactive elements, is paramount. This requires advanced containment and analysis techniques.
- Sophisticated Computational Modeling: Employing advanced computational tools, such as density functional theory (DFT), allows for simulations of atomic behavior, offering insights where direct experimentation is impossible.
- Data Integration and Validation: Combining experimental findings with computational predictions produces comprehensive material models. Rigorous validation ensures model accuracy and reliability.
Predicting Material Properties of Transuranic Elements at 273K
Predicting the properties of transuranic elements (beyond uranium) presents unique challenges due to their radioactivity and limited availability. Their inherent instability and scarcity necessitate indirect approaches:
Computational Modeling: Harnessing the Power of Simulation
Computational modeling using quantum mechanics and DFT calculations is crucial for studying transuranic elements without handling hazardous materials. However, the accuracy of these models depends heavily on the quality of input parameters and the sophistication of the model employed. Dr. Elena Petrova, a theoretical physicist at CERN, notes: "Computational tools, while powerful, are only as good as the data used to inform them."
Extrapolation and Periodic Trends: Inferring from Known Elements
Extrapolating from trends within the actinide series offers a supplementary approach. However, this method assumes consistent periodic trends, which may not always be the case for transuranic elements.
Experimental Data: The Gold Standard
Experimental data, though difficult to obtain due to safety concerns and scarcity, remains the gold standard for validating computational predictions and refining our understanding. Precision experiments on small quantities of transuranic elements, under strict safety protocols, are still crucial.
Iterative Refinement: A Dynamic Process
Predicting transuranic element properties is not a static process; it's an iterative process encompassing computational modeling, data extrapolation, and experimental verification. This cycle of refinement continues to improve predictive accuracy.
Key Takeaways:
- The periodic table is essential for understanding and predicting material properties.
- Addressing knowledge gaps in element properties is crucial for advancing materials science.
- Predictive modeling, particularly for transuranic elements, relies on a combination of computational and experimental approaches.
- An iterative refinement process, incorporating new data and model improvements, is key to advancing our understanding.